Search Catalog Peptides
닫기
Catalog Products for your R&D needs!
PEPTIDES
GMP Peptides
Manufacture of peptide APIs according to GMP regulations.
ANYGEN CO., LTD.'s GMP plant is Korea's first facility specialized in the production of peptide drug raw materials, and has the facilities and infrastructure necessary for the synthesis, purification, drying and quality control of peptide drug raw materials. It has been designed and manufactured to meet the standards of excellent pharmaceutical manufacturing facilities and has been approved by the Ministry of Food And Drug Safety (MFDS) for the first time in Korea to approve the manufacture and quality control of peptide drugs and approval for GMP.
- Our GMP manufacturing
capabilities include -
- Bulk scale solid & solution phase synthesis
- Proportionately scaled HPLC Purification.
- Lyophilization
- Cleanrooms from Class 100,000 to class 10,000
- QA/QC and Regulatory support
- Expertise in all production and purification techniques
- Dedication to quality and GMP compliance
- GMP Plant
-

- GMP Facilities
-
ANYGEN,
Synthesizing the
Cure.
Synthesizing the
Cure.
Wide range from R & D to commercialization

- Synthesis Technology
-
ANYGEN has over 20 years of experience in peptide synthesis and has developed various methods to tailor peptides to the customer’s specific requirements. We carefully select the composition, length of amino acid, scale, and synthesis method based on the customer’s request.
-
Solid-Phase Peptide Synthesis(SPPS)
The technology of choice for manufacturing most peptides up to multi-kg quantities, especially those with longer, more complex sequences. We offer F moc-based solid-phase manufacturing.
-
Solution Phase Peptide Synthesis(SPS)
The technology of choice for manufacturing short peptides or structures that are inappropriate for a resin matrix. This approach ultimately provides a cost-effective process for large-scale manufacture of multi-g to multi-kg lots.
-
Convergent Fragment Synthesis(SPPS + SPS)
This technology, which involves coupling shorter, SPPS generated sequences together in solution, is particularly suitable for some longer peptide structures. Offering higher yields than << Straight-through >> SPPS
-
Solid-Phase Peptide Synthesis(SPPS)

- Purification & Isolation
-
Based on its own developed mass purification technology, it produces high purity products that can be used as raw materials for pharmaceuticals by using industrial HPLC and can be produced in one batch from g scale to kg scale using industrial freeze dryer.
-
Purification
- - 100,000 class clean room and 100 class clean booth
- - Purification system : Column ID 8cm to 20cm Length 50cm
- - Packing Material : ODS C18, 10μm
- - Purification Capacity : 350 ~ 500g/day
- - All systems are validated
-
Isolation(Lyophilization)
- - 100,000 class clean room and 100c class clean booth.
- - One classified isolation suite with lyophilization capacity up to 50kg (ice capacity); gram to multi-kilogram quantities of peptides for 1 bath
- - SUS 316L 15 Tray with over : connected with N2 purge system.
- - All systems are validated.
-
Purification
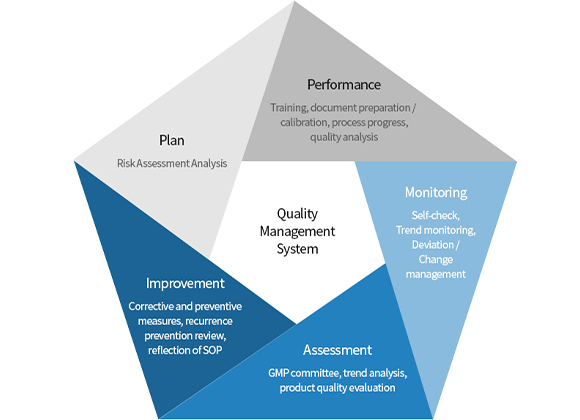
- QA/QC
-
To create the highest quality products through GMP validation, every manufacturing process is monitored, from raw material to synthesis, purification, drying, and before product release, they are inspected on numerous qualifications to ensure the also the safest product.
-
Our standard GMP specifications include
- Appearance
- Solubility
- Identity(via mass spectral analysis, amino acid analysis and HPLC)
- Peptide purity (HPLC)
- Related substances (HPLC)
- Assay (as mass balance)
- Counterion Content
- Moisture content
- Peptide content (by N%)
- Trifluoroacetic acid content
- Chloride content
- Residual organic solvents
- Bioburden
- Endotoxin
- Other API-specific parameters
-
Our standard GMP specifications include
- Analysis Equipment
-
MALDI-TOF Mass, FT-IR, UV/VIS, Polarimeter, Karl Fischer,
HPLC, GC, TOC, Elisa Reader, BSC etc.




